Books, E-books and Journals
-
 European Citizenship through the Lens of EU ValuesBook | 1st edition 2025 | Europe, United Kingdom | Gaëlle Marti€70.00 incl. VATAvailable
European Citizenship through the Lens of EU ValuesBook | 1st edition 2025 | Europe, United Kingdom | Gaëlle Marti€70.00 incl. VATAvailable -
 NATO and International Institutional LawBook | 1st edition 2025 | Europe, United Kingdom | Anne Verhelst€140.00 incl. VATAvailable
NATO and International Institutional LawBook | 1st edition 2025 | Europe, United Kingdom | Anne Verhelst€140.00 incl. VATAvailable -
 European Energy Law Report XVBook | 1st edition 2025 | Europe, United Kingdom | Martha M. Roggenkamp, Catherine Banet€185.00 incl. VATAvailable
European Energy Law Report XVBook | 1st edition 2025 | Europe, United Kingdom | Martha M. Roggenkamp, Catherine Banet€185.00 incl. VATAvailable -
 International Survey of Family Law 2024Book | 1st edition 2024 | World | Robin Fretwell Wilson, June Carbone€115.00 incl. VATAvailable
International Survey of Family Law 2024Book | 1st edition 2024 | World | Robin Fretwell Wilson, June Carbone€115.00 incl. VATAvailable -
 Legal Aspects of Contracts and Third PartiesOn Third-Party Rights, Transfer of Rights, Agency and ContractsBook | 1st edition 2024 | Europe, United Kingdom | Jan Biemans, Lorna Richardson€149.00 incl. VATAvailable
Legal Aspects of Contracts and Third PartiesOn Third-Party Rights, Transfer of Rights, Agency and ContractsBook | 1st edition 2024 | Europe, United Kingdom | Jan Biemans, Lorna Richardson€149.00 incl. VATAvailable -
 Sanctions By and Against International OrganizationsCommon Issues and Current DevelopmentsBook | 1st edition 2024 | World | Giovanna Adinolfi, Alessandra Lang, Chiara Ragni€110.00 incl. VATAvailable
Sanctions By and Against International OrganizationsCommon Issues and Current DevelopmentsBook | 1st edition 2024 | World | Giovanna Adinolfi, Alessandra Lang, Chiara Ragni€110.00 incl. VATAvailable -
 Law of Public-Private Cooperation against Financial CrimeDeveloping Information Sharing to Counter Money Laundering and Terrorism FinancingBook | 1st edition 2024 | Europe, United Kingdom | Benjamin Vogel, Eleni Kosta, Maxime Lassalle€199.00 incl. VATAvailable
Law of Public-Private Cooperation against Financial CrimeDeveloping Information Sharing to Counter Money Laundering and Terrorism FinancingBook | 1st edition 2024 | Europe, United Kingdom | Benjamin Vogel, Eleni Kosta, Maxime Lassalle€199.00 incl. VATAvailable -
 Definition of InsolvencyProposals for Harmonisation in the European UnionBook | 1st edition 2024 | World | Reinhard Bork, Michael Veder, Ben Schuijling€169.00 incl. VATAvailable
Definition of InsolvencyProposals for Harmonisation in the European UnionBook | 1st edition 2024 | World | Reinhard Bork, Michael Veder, Ben Schuijling€169.00 incl. VATAvailable -
 Forcible Protection of CiviliansThe International Legal Framework for Peace OperationsBook | 1st edition 2024 | Europe | Hanna Bourgeois€160.00 incl. VATAvailable
Forcible Protection of CiviliansThe International Legal Framework for Peace OperationsBook | 1st edition 2024 | Europe | Hanna Bourgeois€160.00 incl. VATAvailable -
 Restricting Liberty to Prevent TerrorismThe Belgian and the UK Approaches to Direct Restrictions to the Right to Liberty to Prevent TerrorismBook | 1st edition 2025 | Europe, United Kingdom | Ward Yperman€160.00 incl. VATAvailable
Restricting Liberty to Prevent TerrorismThe Belgian and the UK Approaches to Direct Restrictions to the Right to Liberty to Prevent TerrorismBook | 1st edition 2025 | Europe, United Kingdom | Ward Yperman€160.00 incl. VATAvailable -
 Annotated Leading Cases of International Criminal Tribunals - volume 74The Special Tribunal for Lebanon 31 January 2014 - 14 May 2018Book | 1st edition 2025 | World | André Klip, Steven Freeland€215.00 incl. VATAvailable
Annotated Leading Cases of International Criminal Tribunals - volume 74The Special Tribunal for Lebanon 31 January 2014 - 14 May 2018Book | 1st edition 2025 | World | André Klip, Steven Freeland€215.00 incl. VATAvailable -
 Annotated Leading Cases of International Criminal Tribunals - volume 75The Kosovo Specialist Chambers (KSC) 26 April 2017 – 24 December 2020Book | 1st edition 2025 | World | André Klip, Steven Freeland€215.00 incl. VATAvailable
Annotated Leading Cases of International Criminal Tribunals - volume 75The Kosovo Specialist Chambers (KSC) 26 April 2017 – 24 December 2020Book | 1st edition 2025 | World | André Klip, Steven Freeland€215.00 incl. VATAvailable -
 Directive (EU) 2022/2464 as regards Corporate Sustainability Reporting (CSRD)Texts and CommentsBook | 1st edition 2024 | Europe | Pascal Durand, Abrial Gilbert-d’Halluin€75.00 incl. VATAvailable
Directive (EU) 2022/2464 as regards Corporate Sustainability Reporting (CSRD)Texts and CommentsBook | 1st edition 2024 | Europe | Pascal Durand, Abrial Gilbert-d’Halluin€75.00 incl. VATAvailable -
 The Revival of the Rule of Law IssueBook | 1st edition 2024 | World | Marek Safjan€199.00 incl. VATAvailable
The Revival of the Rule of Law IssueBook | 1st edition 2024 | World | Marek Safjan€199.00 incl. VATAvailable -
 Property Law Reform, Sustainability and the CommonsBook | 1st edition 2024 | Europe, United Kingdom | Vincent Sagaert, Dorothy Gruyaert, Marie-Laure Degroote, Kato De Schepper, Vincent Janssen, Flore Vavourakis€160.00 incl. VATAvailable
Property Law Reform, Sustainability and the CommonsBook | 1st edition 2024 | Europe, United Kingdom | Vincent Sagaert, Dorothy Gruyaert, Marie-Laure Degroote, Kato De Schepper, Vincent Janssen, Flore Vavourakis€160.00 incl. VATAvailable -
 Transitional Justice, Impunity and the Judicialization of PoliticsBook | 1st edition 2025 | Europe, United Kingdom | Michael Humphrey, Estela Valverde€125.00 incl. VATAvailable
Transitional Justice, Impunity and the Judicialization of PoliticsBook | 1st edition 2025 | Europe, United Kingdom | Michael Humphrey, Estela Valverde€125.00 incl. VATAvailable -
 Customs and DutiesBook | 1st edition 2024 | Europe | Kerstien Celis, Josse Verbeken€80.00 incl. VATStudent price: €35.00Available
Customs and DutiesBook | 1st edition 2024 | Europe | Kerstien Celis, Josse Verbeken€80.00 incl. VATStudent price: €35.00Available -
 The Marburg Group’s Comments on the European Commission's Parenthood ProposalBook | 1st edition 2024 | Europe, United Kingdom | Christine Budzikiewicz, Konrad Duden, Anatol Dutta, Tobias Helms, Claudia Mayer€69.00 incl. VATAvailable
The Marburg Group’s Comments on the European Commission's Parenthood ProposalBook | 1st edition 2024 | Europe, United Kingdom | Christine Budzikiewicz, Konrad Duden, Anatol Dutta, Tobias Helms, Claudia Mayer€69.00 incl. VATAvailable -
 The Role of Legal Precedent in Private LawA Comparative StudyBook | 1st edition 2024 | World | Christina Ramberg€130.00 incl. VATAvailable
The Role of Legal Precedent in Private LawA Comparative StudyBook | 1st edition 2024 | World | Christina Ramberg€130.00 incl. VATAvailable -
 Annotated Leading Cases of International Criminal Tribunals - volume 73International Criminal Court (ICC) 8 March 2018 - 8 June 2018Book | 1st edition 2024 | World | André Klip, Steven Freeland€215.00 incl. VATAvailable
Annotated Leading Cases of International Criminal Tribunals - volume 73International Criminal Court (ICC) 8 March 2018 - 8 June 2018Book | 1st edition 2024 | World | André Klip, Steven Freeland€215.00 incl. VATAvailable
Catalogue 2023
We are delighted to present our new and forthcoming publications in this 2023 catalogue.
We continue to publish a strong portfolio of books that explore topical issues and contribute meaningfully to their respective fields.
Browse the catalogue to find out more about the exciting projects we are working on!
Conferences
Larcier-Intersentia warmly invites you to come and meet us at the conferences, book fairs and other academic meetings, which will be attended throughout this year.
You will be able to review our latest publications and enjoy attractive discounts on all publications on display.
If you work on a script yourself, we would be very interested in exploring publishing opportunities with you as we always seek opportunities to expand our publishing program.
E-book collection
We have made over 200 books available digitally for both individuals and institutions.
Institutions can access our e-books via Cambridge University Press's academic digital publishing platform, Cambridge Core. Cambridge Core is the online home of over 34,000 academic books and over 370 journals.
Individuals can purchase individual e-books via our website.
New from IntersentiaOnline

Enterprise Foundation Law in a Comparative Perspective (Open Access)
Many large companies – like Bosch, Ikea and Novo-Nordisk – are owned by enterprise foundations. This book provides an overview of enterprise foundation law in six European countries and the US. The book explores enterprise foundation law in the seven aforementioned nations and analyzes how the law influences the prevalence and governance of enterprise foundations around the world.
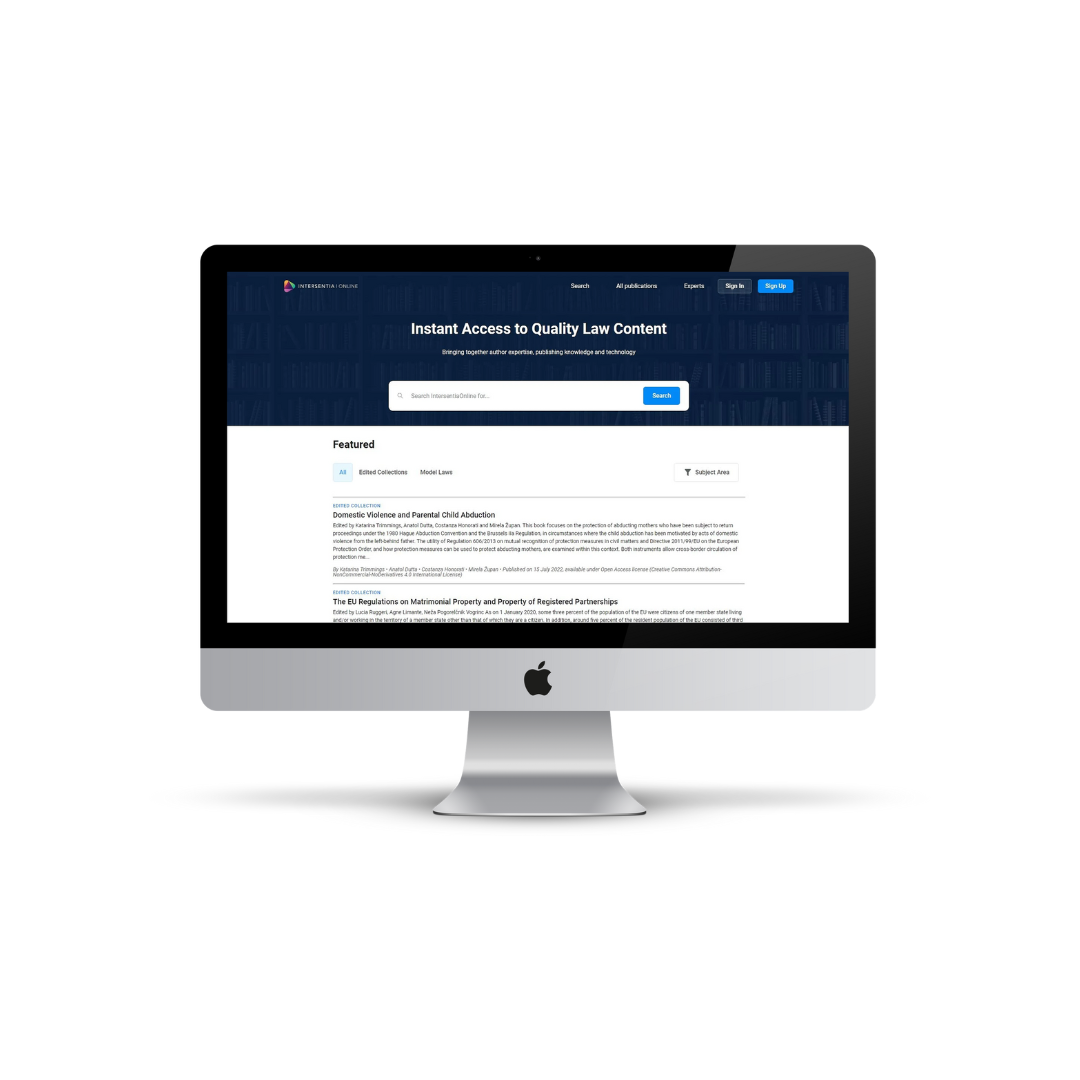
IntersentiaOnline is a new online platform that provides instant access to quality law resources. By combining technology, publishing knowledge and expert authors, this platform can encompass resources that are quality-assured, topical and updateable. The platform includes both Open Access and paid for content, in a range of formats: articles, monographs, edited collections, model laws, case commentaries and more.
Interviews


Author Speaking | Lucia Elena Arantes Ferreira Bastos | Part 2
In this exclusive interview, we speak to Lucia Bastos about her book Transitional Justice in Brazil: Walking the Tightrope, published as part of Intersentia’s Series on Transitional Justice. The second part of this interview discusses human rights issues in Brazil, how the state has sought to correct these historical abuses and in what ways these policies must be adapted to carry out the goal of transitional justice. Read more.


European and International Law
We interviewed Laurence Burgorgue-Larsen on this recently published book. She discusses the meaning of “Plurality” and “Diversity,” and examines how these two legal and political concepts are affected by the burdens of the past: colonialism, war and the traditional attitudes of the countries addressed. Read more.


Author Speaking | Lucia Elena Arantes Ferreira Bastos | Part 1
In this exclusive interview, we interview Lucia Bastos about her book Transitional Justice in Brazil: Walking the Tightrope. The first part of this interview discusses the political history of Brazil and the cultural and legal implications of holding the past dictatorship to account. Read more.
1500 specialist and renowned authors at your service
Reliable payment partners


